The Brains On Water Detective Handbook
Dissolved Oxygen
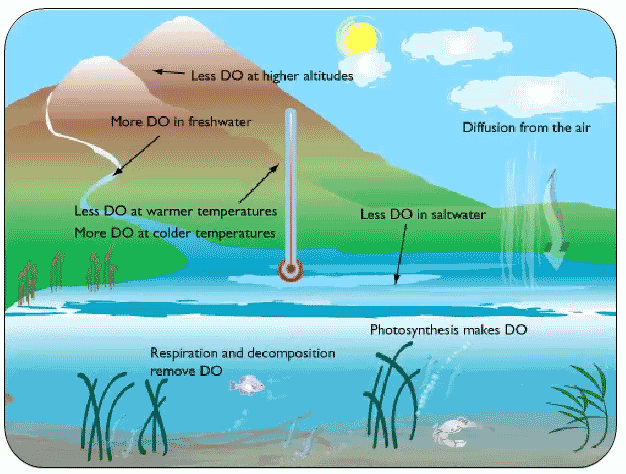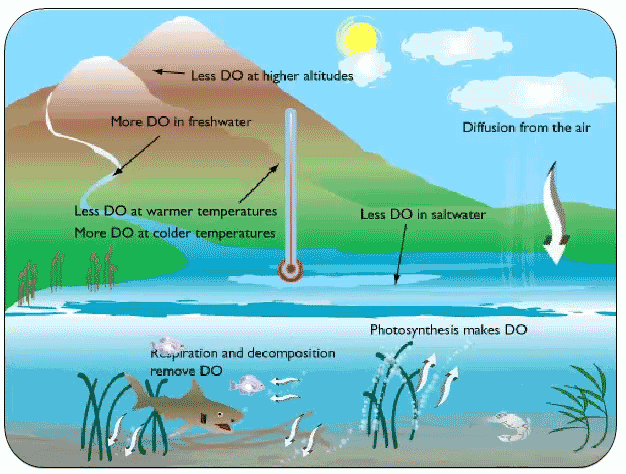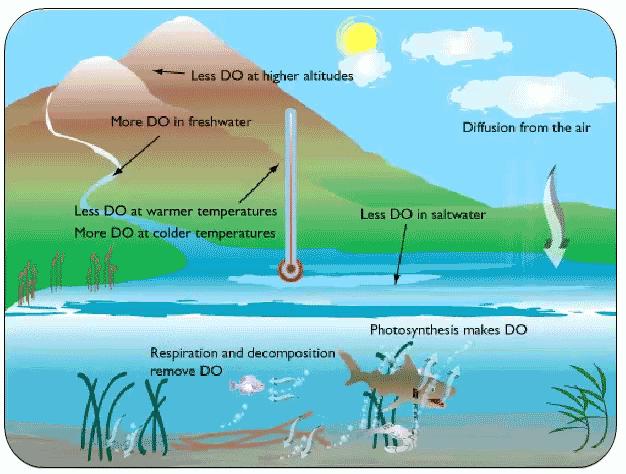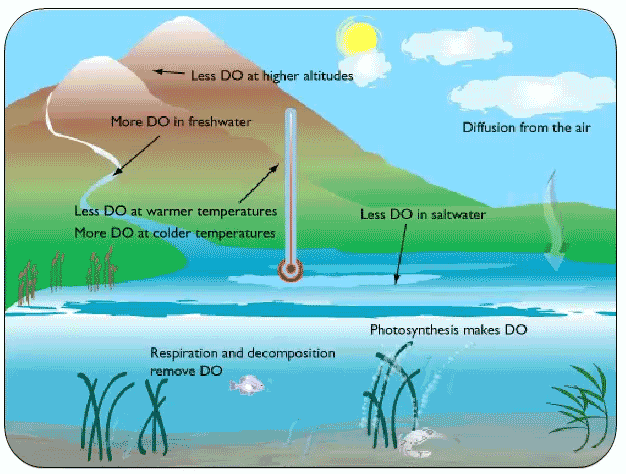
We all know that land-loving animals need oxygen, but so do most water dwelling, or aquatic, animals. Carp, crayfish and clams all need oxygen to survive. There are two ways oxygen can get into water: from the air, and from oxygen produced by underwater plants. When oxygen dissolves in water and can be used by aquatic organisms to breathe. We call this dissolved oxygen (or DO for short). Dissolved oxygen is extremely important for the health of aquatic ecosystems - it is one of the best ways to figure out how suitable a stream or lake is for supporting life. Bodies of water with higher levels of dissolved oxygen can support many different kinds of aquatic organisms. It’s harder for creatures to survive when there are low levels of DO, and when DO is extremely low, plants and animals can die.
How Nature Affects Dissolved Oxygen levels
Time of day: Dissolved Oxygen levels change depending on the season. DO levels also change over a 24 hour period thanks to aquatic plants. Plants convert sunlight and the gas carbon dioxide (CO2) into energy by a process called photosynthesis. One of the byproducts of photosynthesis is oxygen. So, during the day, as aquatic plants photosynthesize to make their energy, they are also releasing oxygen into the water. In the evening, plants actually breathe back in some of that oxygen. That’s why DO levels are often highest during the day and lower at night.
Temperature: The temperature of water also changes how much oxygen it can hold. Ever notice how really cold pop is more fizzy than warm pop? That’s because the temperature of a liquid determines how much gas can be dissolved in it. This means colder water can hold more dissolved oxygen.
Salt: Salt can also affect Dissolved Oxygen. The saltier the water, the less DO it can hold. This is why saltwater tends to have less DO than freshwater, all else being equal.
% Saturation: To see how healthy a body of water is, it’s important to measure the DO and the temperature. This allows you to calculate something called the percentage (%) saturation. That’s a fancy way of saying how much DO water is holding.
Remember, a higher percentage is generally considered good.
How Humans Affect Dissolved Oxygen Levels
Stuff like factories, power plants and even farms can affect DO levels. They do this by messing with the temperature or saltiness of a body of water. They can also affect the plants in the water that help produce oxygen.
Things that affect the temperature of water:
Urban runoff: City streets are covered in material known as asphalt. Asphalt is warmer than natural surfaces, so water that falls on city streets and runs off into a river or lake can be warmer than normal. This can heat up the body of the water, hurting its ability to hold dissolved oxygen.
Lack of tree cover:
Trees help cool bodies of water by providing shade near the shore. When there are few or no trees, a body of water can heat up faster hurting its ability to hold DO. On the other hand, if there are too many trees there may not be enough light hitting the water and the aquatic plants could have trouble.
Erosion:
Hillsides and riverbanks break down over time. As they do, bits of dirt, debris and plant particles crumble and fall into bodies of water. We call this natural process erosion. If there is too much erosion too quickly, water can get cloudy with all this debris and that could decrease the light reaching the aquatic plants. When the water plants can’t get light they can’t photosynthesize and make oxygen for the water creatures living around them.
Salt:
In winter, people use salt on roads to help melt snow and ice. However, this salt often ends up going into waterways as well, which increases the saltiness, or salinity, of a body of water. The salties a body of water is, the less dissolved oxygen it can hold.
Too Many Nutrients:
Plants need more than just sunlight and the gas carbon dioxide, to survive. They also need things like nitrogen and phosphorous. These are chemical elements called nutrients. These are often found naturally in soil. However, sometimes humans use large amounts of these nutrients when farming. These extra nutrients can get washed into bodies of water over time. At first, this might help plants by feeding them and thereby increasing the amount of dissolved oxygen. However, this can also lead to way too many plants growing in a specific area. This disturbs other animals and messes with dissolved oxygen levels.
There are other ways large amounts of nutrients end up in water. Sometimes factories produce nutrient rich waste that ends up in lakes or streams. Pet waste is also chock-full of nitrogen and phosphorous and all that can be carried to your water after a rainstorm. Plus, pet waste sometimes has bacteria like E. coli which is dangerous to people. So don’t let this get into our water, be sure to pick up after your pet!
What does my reading mean?
If you have a high DO reading, you’re in the clear! Your water body can support a diversity of aquatic life. Look around and try to see what natural features might be helping keep the water healthy.
If you have a low DO reading, the water is less likely to be able to support a diversity of aquatic life. Take a look at the factors outlined above and see if any might be contributing to your reading.
Temperature
Temperature has a big effect on the plants and animals living in a body of water. Consider us humans. While we’re pretty good at adapting to swings in temperature, there are specific ranges in which we function best. Imagine having to play a championship soccer game in -10 ̊ F cold, or take an important test for school if it’s over 100 ̊ F. We just don’t work as well. Its the same for aquatic life. Some organisms are more sensitive than others. For example, brook trout thrive in waters 55-65 ̊ F , but temperatures above 78 ̊ F are fatal. But fish like the bluegill sunfish can survive in waters as warm as 90 ̊ F!
The temperature of water also affects levels of Dissolved Oxygen. The colder the water, the more dissolved oxygen it can hold. If water gets too warm, there may be a surge in plant growth which can eventually lead to problems for the body of water as well. Rapid changes in temperature can also make fish and insects more vulnerable to parasites, disease, and the harmful effects of pollutants.
How Nature Affects Water Temperature
There are lots of natural factors that influence the temperature of your water. Sunlight and heat from the atmosphere affects the temperature of water. So in sunnier, warmer places, like around the Earth’s equator, water is usually warmer than it is in colder, cloudeir places, like the North and South poles.
The size and depth of a water body also affects its temperature. Bigger, deeper bodies of water tend to be cooler than smaller, shallower water. Moving water like streams or rivers also tend to be cooler than water that stays put.
Since water temperature can change so much, there’s no one-size-fits-all temperature that’s too hot, too cold, or just right. So how do you know if a body of water near you is a good temperature? Well, you can measure the temperature, share your data with XXX and see how it compares to other spots nearby.
How Humans Affect Water Temperature
Runoff: Sometimes a body of water heats up because some extra source of heat is added to it. This is called “thermal pollution”. One of the biggest sources of thermal pollution is called runoff. This is water that drains to a lake or river from somewhere like a factory or city. Factories sometimes heat up water to help them make things and then they dump that water back into a lake or river when they are done. Sort of like how you might heat up a pot of water to cook spaghetti then dump it after the noodles are soft. Warm rainwater runoff can also come from city streets which are often warmer than natural ground. When rain lands, it soaks up that heat and carries it to a nearby lake or river.
Dams: Dams can also cause water temperature to increase. That’s because dams block or slow down the flow of water. If the water isn’t moving it heats up more easily.
Erosion: Another source of thermal pollution is erosion. Erosion is the gradual breakdown or crumbling of the land around a water body. Dirt and debris created by erosion can end up in our waterways. All this extra stuff increases the turbidity -- or cloudiness of the water. The more turbid, the more stuff there is to absorb the energy of the sun and heat up the water.
Lack of tree cover: Cutting down trees, known as “deforestation,” can also cause temperature increases. Natural shade from trees and other vegetation helps keep waters cooler. If trees have been cleared, this could cause water to be more exposed to the sun’s warming rays.
Turbidity
When you look at water, one of the first things you notice is how clear it is. Water clarity is one of the most recognizable signs of water quality. Generally, clear water is healthier than murky water. Water quality specialists have a special word for describing how clear a water body is - turbidity. Turbidity is a measure of how much water is clouded by floating particles like clay, silt, algae, or sewage. More floating particles equals greater turbidity. This floating material reduces the amount of light that can penetrate through the water.

Aquatic plants need sunlight to make energy in process. It’s a process called photosynthesis. So if the water is very turbid, or cloudy, it can weaken aquatic plants, which in turn reduces the amount of dissolved oxygen they produce. To make matters worse, all of these floating particles can easily heat up by absorbing sunlight, causing the body of water to get warmer. Warner water doesn’t hold oxygen as well as cold water and plants and animals need plenty of oxygen to survive.
Plus, turbid water can make life tough for fish and other aquatic organisms since the extra material clouds their vision or messes with their sense of smell. Imagine how annoying it would be to have to wade through a bunch of junk just to find a snack in the kitchen! Extremely high turbidity can even impair gill function in fish. Gills are special organs that help fish breathe, so they are super important.
Measuring turbidity before, during, and immediately after a rainstorm can also be very informative. If you see a big jump in turbidity after a rain, that’s a good indication that there may be a runoff problem.
How Nature Affects Water turbidity
Not all water is naturally 100% clear. Fast-moving streams or rivers might kick up more sediment, making them naturally more turbid. Normal erosion can contribute to turbidity as well.
How Humans Affects turbidity
Erosion: A major source of dirt and plant particles in water comes from soil erosion. That’s when bits of dirt, debris and plant matter get loose. These little bits can be washed into rivers and lakes leading to cloudy water.
Some degree of erosion over time is natural, but human activity can speed up the process. For instance, farms and constructions sites often create a lot of extra dirt and debris. Also, when humans cut the trees around a lake or a river, they are removing plants that helped keep the soil together. Without those plants and their roots it’s easier for the soil to be washed into the water during a rainstorm.
Urban runoff: Modern life produces lots of little bits of stuff, which can get carried into natural waterways by rain or snowmelt.
Algae blooms: Algae is a simple, non-flowering plant that can grow in water. Nutrients from farms can run off into a body of water and feed algae leading to an overgrowth of the stuff. When too much algae grows on a pond or lake it can cloud the water and lead to problems.
Disturbances: When there are too many “bottom-feeder” animals in a body of water, like carp or catfish, it can lead to problems. That’s because these fish stir up sediment on the river or lake floor. Boats can do the same thing. In both cases the result is cloudy water.
pH Levels
Did you get a high turbidity value? Look around - is there a big construction project nearby, or farm? Is your body of water protected by surrounding plant life like trees, or is it bare dirt, or pavement? What color is the water? Is it greenish? That could indicate excessive algae growth. Or is it brownish dirty, indicating sediment runoff? Or perhaps tea colored, which could indicate tannins coming from decaying organic matter?
pH is a measure of how acidic or basic a solution is. Acidic water contains extra hydrogen ions (H+) and basic water contains extra “hydroxyl” ions (OH-). pH is measured on a scale, from 0-14. 1 is very acidic and 14 is very basic. 7 is in the middle and means a substance is not acidic or basic but instead neutral.

Lemon juice is very acidic with a pH level of 3. Your stomach juices are also very acidic, with pH levels of 1. On the other side there are things like bleach and ammonia, which are very basic. Pure water should have a pH of about 7, meaning it is neutral. Sea water is slightly more basic than drinking water, while rain and snow are slightly more acidic.
A change of 1 unit on a pH scale seems small, but the pH scale is a logarithmic scale. This means that a change in 1 unit represents a 10 fold, or 10 times bigger/smaller change in the pH. That water with pH of 6 is 10 times more acidic than water with a pH of 7, and water with a pH of 5 is 100 times more acidic than water with a pH of 7.
Just like animals and plants are adapted for specific temperature ranges, they’re adapted for specific pH ranges too. A pH below 4 or above 10 will kill most fish and very few animals can tolerate waters with a pH below 3 or above 11. If the water becomes more acidic than a plant or animal is used to, it can hurt them in lots of ways. It might mess with how their eggs develop, or impair their gills, or damage their skin. Amphibians are especially sensitive because their skin is so porous, or easily able to absorb things from the environment. Changes in pH are generally a pretty good indicator that something harmful might have entered a body of water.
The pH level of water can also affect how harmful other stuff in that water is for animals. Heavy metals, for example, become more toxic if the water is more acidic. pH levels can also affect how easily certain chemicals dissolve in water.

How Nature Affects pH levels
pH can vary naturally depending on the environment the water is in. Sandy soils tend to be more acidic, while chalky soils more basic. If your water is surrounded by pine trees, the needles can leech into the water making it more acidic. Rain and snow is also slightly acidic, so recent rainfall or snowmelt can temporarily change the pH of your water.
How Humans Affects Water pH levels
Runoff: Mining can create acidic runoff that flows into waterways. Factories also sometimes release runoff that is high or low on the pH scale. Pollution from waste pipes or agricultural sources can feed into waterways and affect pH. Industrial processes that involve lots of soaps or detergents can cause pH to increase, while those that use acids can decrease pH.
Acid Rain: Certain air pollution can make water vapor in the atmosphere even more acidic, causing so-called acid rain to fall and decrease the pH of waterways.
Salt: When it snows, people often use salt to help things melt. However, this salt can end up in our waterways. Salt tends to increase pH levels.
Removal of natural defenses: Wetlands, marshes and swamps are good at helping protect nearby bodies of water from big changes in pH. Even a buffer of trees and plants around a body of water can make a big difference
If your pH is in the 5-8 range you’re water is pretty normal. If it’s less than 5 or greater than 8, your water may have an issue. Consider the factors above and whether one of them could be contributing to your reading.